In the early morning hours of Feb. 17, three Woodside siblings piled into their mom's Acura for a seven-hour, 445-mile drive to Banning, California. For the teens, who'd been stuck at home for nearly a year during the pandemic, the trip had a special purpose: to be part of Moderna's landmark vaccine trial.
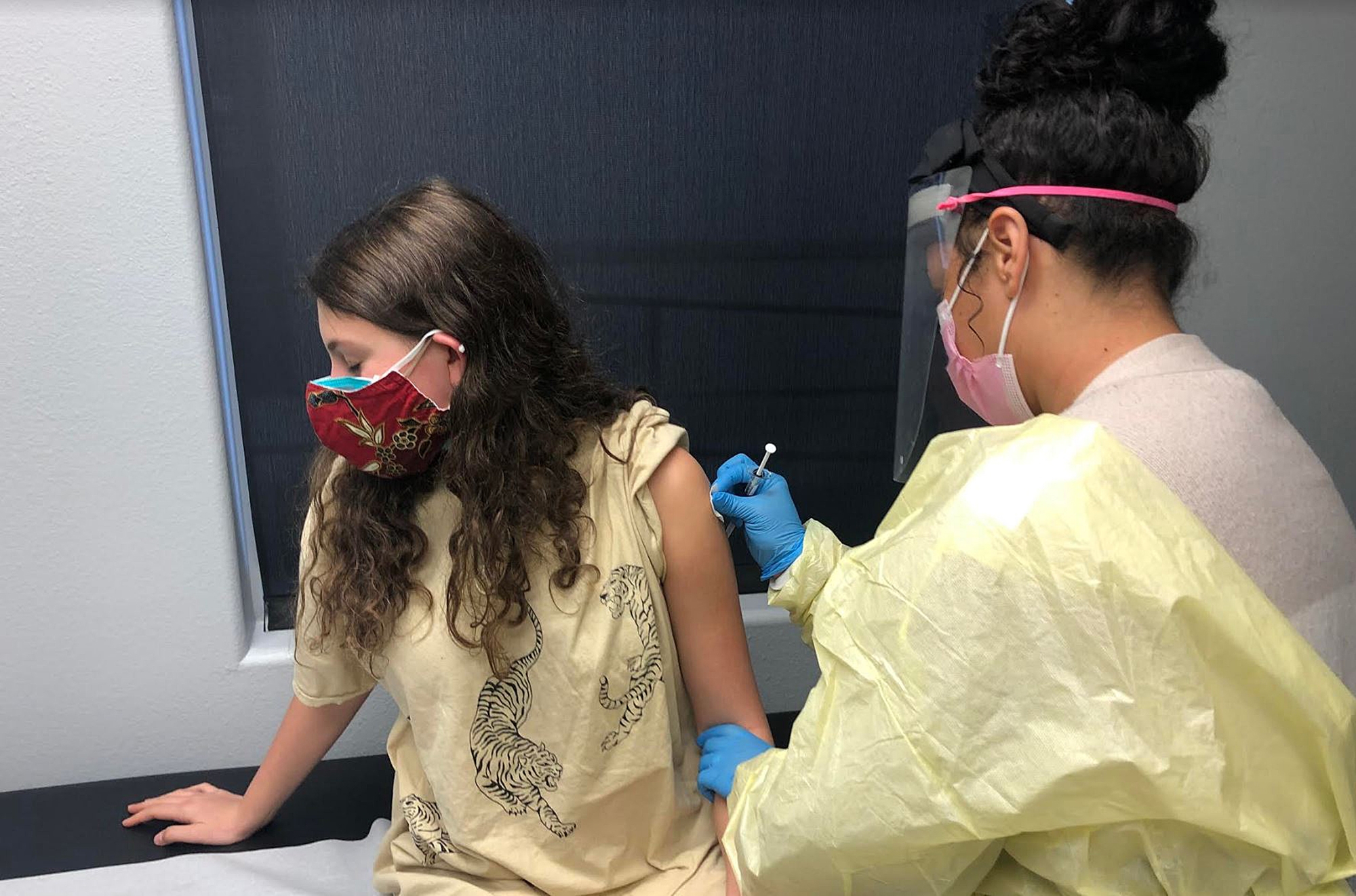
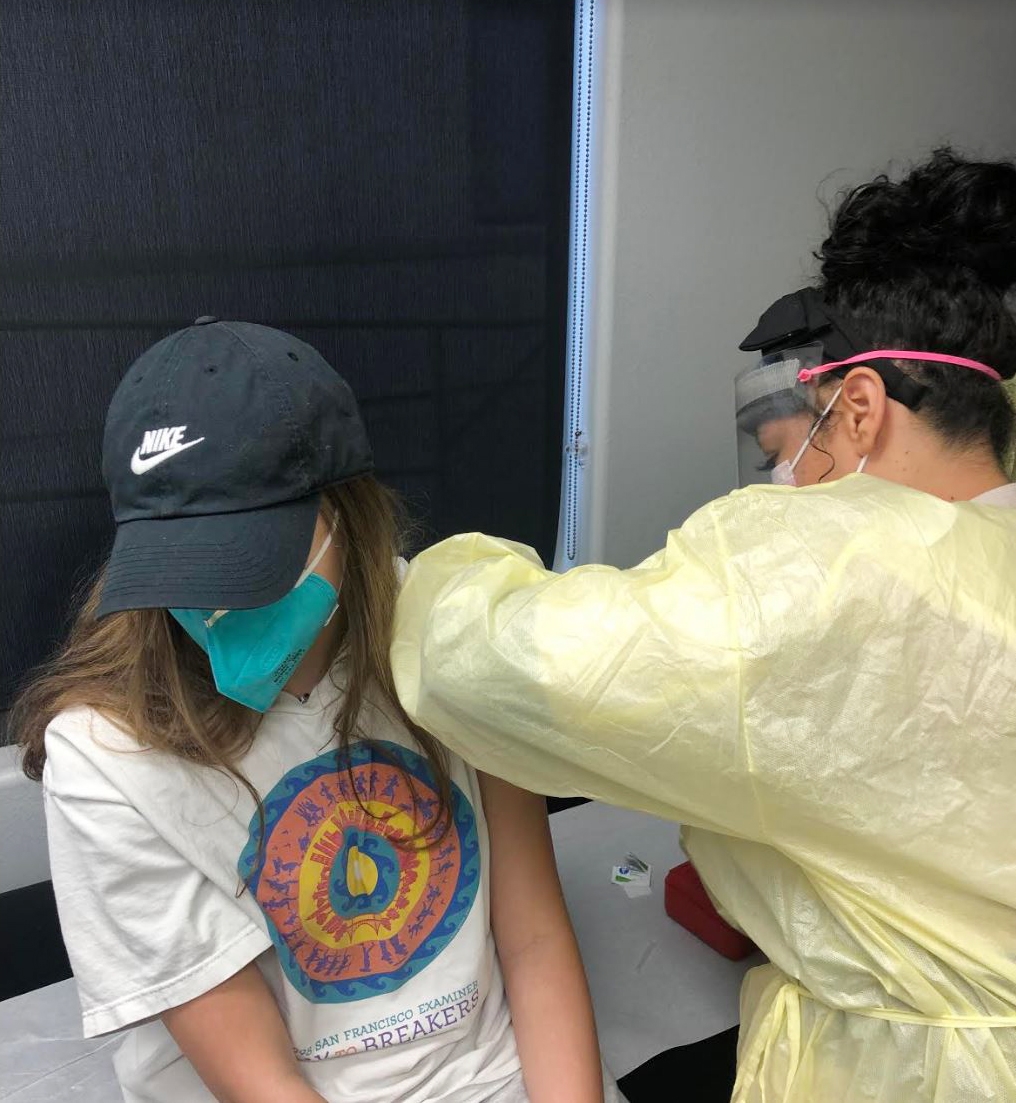
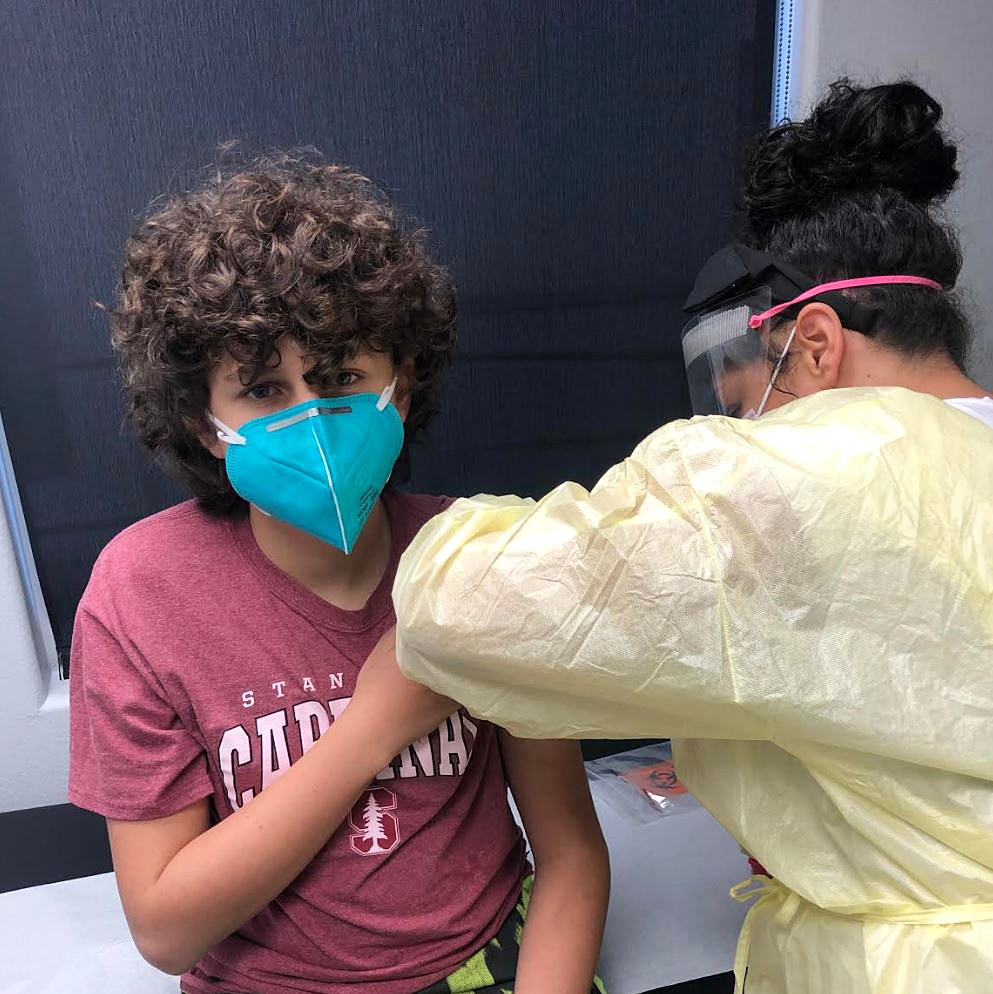
Interested in receiving the COVID-19 vaccine early, the Helfand siblings, Adin, 17, Morgan, 15, and Ben, 12, applied to take part in the study and were accepted.
"It was exciting to have some sort of change," said Morgan, a sophomore at Woodside High School, about their trip down to Southern California. "Up until the trial, we had not gotten on an airplane (since the pandemic began). It was a reason to get out of our small hometown. It was a window into the future and being able to exit quarantine."
With '90s music playing on Spotify, the teens arrived at Velocity Clinical Research facility for the first part of the trial after stopping for lunch in Pasadena. Researchers recorded their weight and height, took their blood pressure and temperature, then took a blood sample and COVID-19 nasal swab test. They signed consent waivers, then received their first of two jabs (the second would come 28 days later). They waited for an hour to see if they had reactions to the shots.
After a pit stop at In-N-Out, they were home by 1 a.m.
"The benefit of being in the teen trial outweighs the detriments of a long drive," said Adin, a junior at Woodside High. "All of that was worth it because you get to be part of something larger than yourself."
The ongoing Moderna teen study, dubbed "Teen Cove Study," includes 3,000 participants ages 12 to 17 and began in December 2020, according to the U.S. National Library of Medicine. Participants have to have never tested positive for COVID-19. Some 67% of those in the trial received the study vaccine and the remaining 33% were given a placebo, according to the pharmaceutical company.
Moderna wants to generate data sometime this spring, so adolescents can be vaccinated before the 2021-22 school year begins this fall, according to a press release. Once the vaccine is approved by the FDA, the Helfand siblings will be able to receive the vaccine, if they haven't already.
The experiment is double-blinded, meaning neither the participants nor those administering the vaccinations know who is receiving a particular treatment, so the three still don't know which of them received the placebo, a saline shot, or the study vaccine. Morgan said she thinks she may have gotten the vaccine because she had cold symptoms (a headache, fatigue and slight fever) as well as some redness at the injection site after receiving her second shot in March. Her siblings did not have any symptoms after their second shots.
Using an app called Patient Cloud, they would record how they were feeling, how their injection sites looked and temperature. Once a week, the researchers would call them to check if they had any symptoms.
The three, whose mother is a doctor, said they wouldn't hesitate to take part in another study down the line.
"I learned that being a part of the future of science is really cool," Morgan said. "It opened my eyes to have doctors who were able to create vaccines to be able to combat something like this. It's a way to be able to fix the pandemic; it's the least I can do is take part (in the study)."
Adin said taking part in the trial felt like almost a service to the medical community.
"You are in essence putting yourself at risk," she said. "We knew we were not going to be taking crazy sorts of risks (since the vaccine had already been tested on adults for nearly a year). But it opened my eyes to the whole process of getting drugs and medicine approved. They (researchers) really just want to make sure everyone still stays healthy and the product is helpful and viable."
The teens hope the vaccines, once approved for all age groups, can bring society back to a normal. Based on evidence from clinical trials, the Moderna vaccine was 94.1% effective at preventing COVID-19 in people ages 18 to 95 years old who received two doses who likely hadn't previously been infected with the virus.
"You should just do it (get the vaccine), after that you'll be way safer for COVID," Ben, a seventh grader at Woodside Elementary School, said. "You're not the lab rat." Nearly 30,000 people took part in Moderna's adult COVID-19 vaccine trial in the U.S.
Morgan said she urges anyone presented with the chance to be vaccinated to take it to help reach herd immunity and protect others from contracting the virus. Morgan's reaction from the shot she received lasted fewer than 24 hours and she said she has no lasting side effects, unlike some people who become sick with the virus.
Adin can't wait to get back to normal teen activities such as prom and even in-person Advanced Placement tests.
"We come from a very privileged background and some kids don't have access to the resources we do; they were hit harder (by the pandemic)," she said. "Another bonus, if we can get the vaccine available to children, we can return back to schooling and access to equitable learning."
Pfizer has been studying the use of its vaccine in youth ages 12 to 15 years old.The drug company said Wednesday, April 7, that it had 100% efficacy against severe cases of the disease in this age group. Last week, Pfizer gave the first doses of its COVID-19 vaccine to children 5 to 11 years old.
For more information on the Moderna vaccine (approved for those 18 years old and older) and the two other FDA-approved COVID-19 vaccines: Pfizer (approved for those 16 years old and older) and Johnson & Johnson (approved for people over 18 years old), go to the CDC's website.
Every Californian over 16 years old became eligible for vaccines on April 15.
Find comprehensive coverage on the Midpeninsula's response to the new coronavirus by Palo Alto Online, the Mountain View Voice and the Almanac here.



Comments